Emergency use of vaccine only if \"No adequate, approved, and available alternative\" (such as vitamin D)
What is an Emergency Use Authorization (EUA)? (FDA)
An Emergency Use Authorization (EUA) is a mechanism to facilitate the availability and use of medical countermeasures, including vaccines, during public health emergencies, such as the current COVID-19 pandemic. Under an EUA, FDA may allow the use of unapproved medical products, or unapproved uses of approved medical products in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions when certain statutory criteria have been met, including that there are no adequate, approved, and available alternatives . Taking into consideration input from the FDA, manufacturers decide whether and when to submit an EUA request to FDA.
If the FDA admitted that there are indeed proven alternatives (Vitamin D, Ivermetin, etc), it seems that the FDA would have have stop emergency use authorizations for the (profitable) vaccines
Vitamin D
COVID-19 treated by Vitamin D - studies, reports, videos
{include}
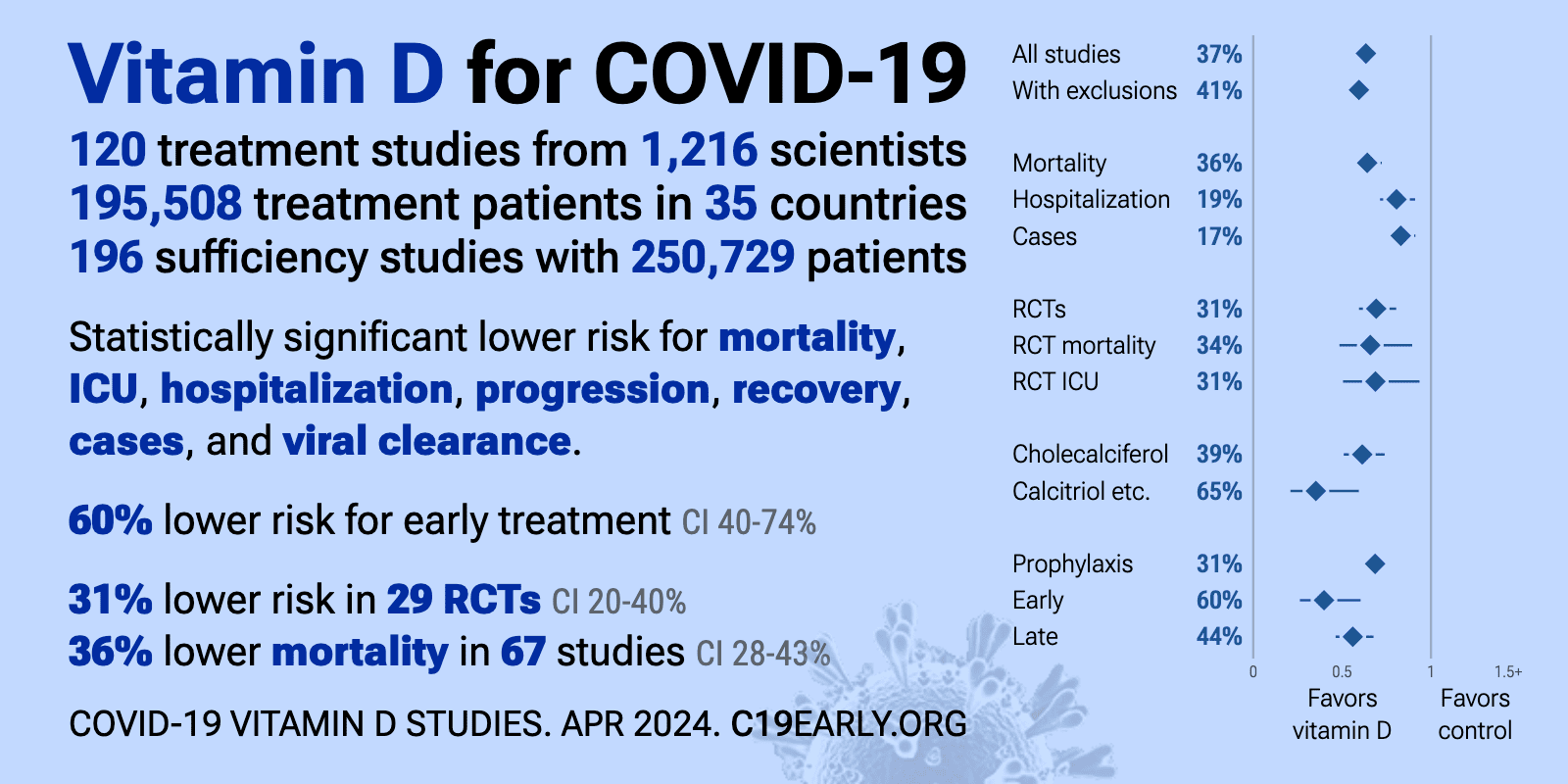
- The above image is automatically updated
26 health factors increase the risk of COVID-19 – all are proxies for low vitamin D
