US govt. notices new Vitamin D proof, will wait years for large trials to end
Vitamin D: Moving Forward to Address Emerging Science
Nutrients 2017, 9,1308; doi:10.3390/nu9121308
Christine L. Taylor h* TaylorCL3@od.nih.gov, Christopher T. Sempos 1, Cindy D. Davis 1 and Patsy M. Brannon 2
Office of Dietary Supplements, National Institutes of Health, Room 3B01, 6100 Executive Boulevard, Bethesda, MD 20892, USA; SemposCH@nih.gov (C.T.S.); DavisCI@nih.gov (C.D.D.)
Division of Nutritional Sciences, 225 Savage Hall, Cornell University, Ithaca, NY 14853, USA; pmb22@cornell.edu
📄 Download the PDF from VitaminDWiki
The science surrounding vitamin D presents both challenges and opportunities.
Although many uncertainties are associated with the understandings concerning vitamin D, including its physiological function, the effects of excessive intake, and its role in health, it is at the same time a major interest in the research and health communities. The approach to evaluating and interpreting the available evidence about vitamin D should be founded on the quality of the data and on the conclusions that take into account the totality of the evidence. In addition, these activities can be used to identify critical data gaps and to help structure future research. The Office of Dietary Supplements (ODS) at the National Institutes of Health has as part of its mission the goal of supporting research and dialogues for topics with uncertain data, including vitamin D. This review considers vitamin D in the context of systematically addressing the uncertainty and in identifying research needs through the filter of the work of ODS. The focus includes the role of systematic reviews, activities that encompass considerations of the totality of the evidence, and collaborative activities to clarify unknowns or to fix methodological problems, as well as a case study using the relationship between cancer and vitamin D.
Clipped from PDF
Most sources of Vitamin D data have been explored
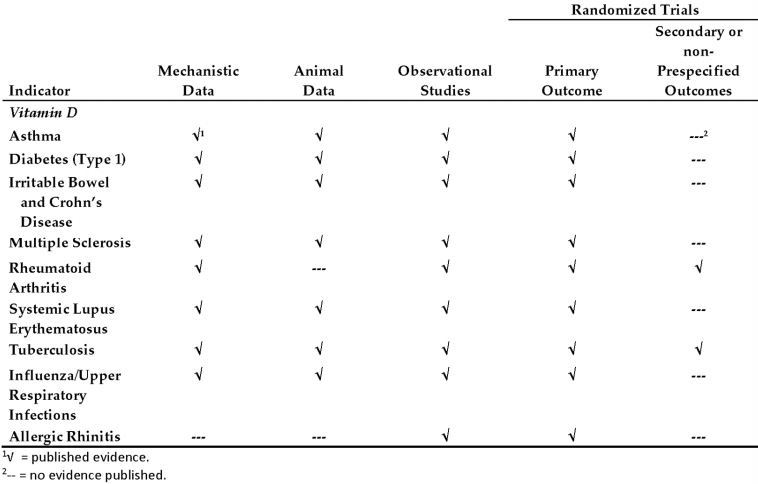
2010 version of the table
Note: more data filled in and there should be 20+ more rows of diseases in 2017
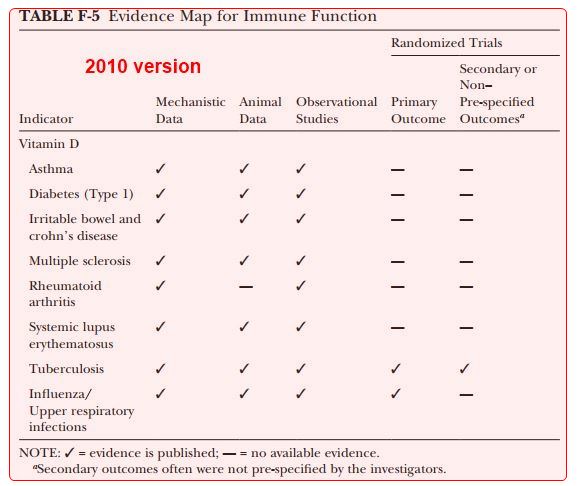
Table 7. Current Randomized-Controlled Trials with >10,000 Participants