Risk of Autoimmune Disease increases as you age

Perplexity AI Dec 2025
Executive Summary
Autoimmune diseases demonstrate a sharp inflection point after age 50, with prevalence rates increasing from approximately 35 per 1,000 in the 40-49 age bracket to 65 per 1,000 in the 50-59 cohort—a near doubling within a single decade. This trajectory continues exponentially, reaching 180 per 1,000 by age 80+. The phenomenon reflects a confluence of immunosenescence, hormonal transitions, and cumulative environmental exposures that fundamentally restructure immune regulation. Women face disproportionate risk, comprising 63% of autoimmune disease cases, with menopause serving as a critical biological accelerator. Understanding these mechanisms provides the foundation for targeted surveillance and intervention strategies in high-risk populations.1 2 3 4
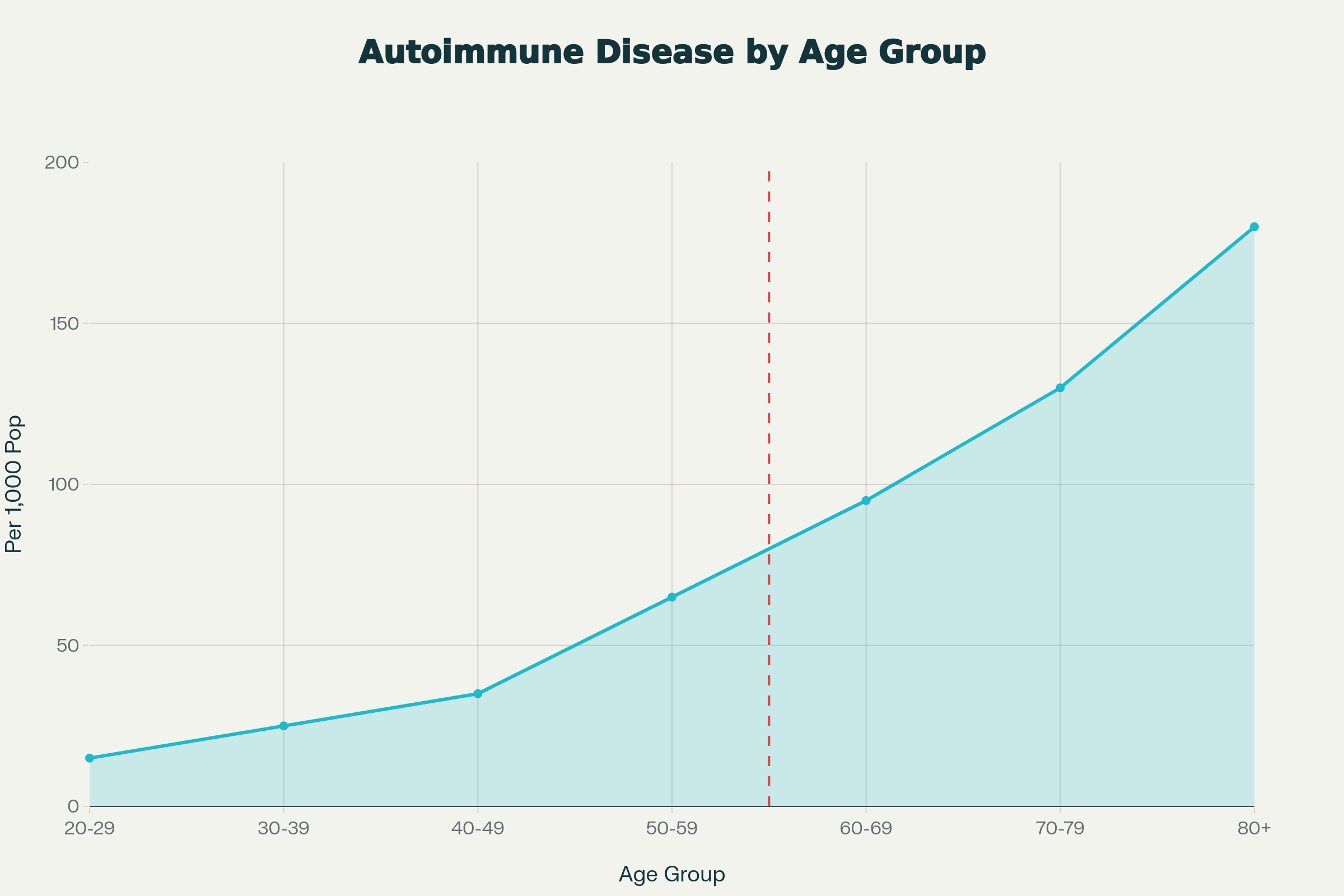
Autoimmune Disease Prevalence by Age Group: Sharp Increase After Age 50 Threshold
1. Epidemiological Landscape: The 50-Year Threshold
Quantifying the Age-Related Surge
Population-level data reveal a distinct epidemiological pattern. A Mayo Clinic study analyzing 10 million electronic health records documented that 9% of individuals develop autoimmune diseases from age 50 onward, with incidence climbing steeply after 65. Mortality data corroborate this trend: systemic autoimmune diseases (SAIDs) show an exponential increase in death rates, reaching 453.9 per 1 million population in women over 80 compared to just 1.8 per 1 million in those under 40.2 4
The National Health and Nutrition Examination Survey (NHANES) demonstrates that autoantibody prevalence rises from 10% in younger adults to 25% in older persons, translating to 41.5 million affected individuals over 60. This represents a 150% increase in biomarker positivity, often preceding clinical disease manifestation by years or decades.5
Table 1: Age-Specific Autoimmune Disease Prevalence and Mortality
| Age Group | Prevalence (per 1,000) | Mortality Rate (per 1 million) | Female:Male Ratio |
|---|---|---|---|
| 20-29 | 15 | 0.8 | 1.1:1 |
| 30-39 | 25 | 1.1 | 1.2:1 |
| 40-49 | 35 | 8.5 | 1.5:1 |
| 50-59 | 65 | 13.9 | 1.8:1 |
| 60-69 | 95 | 82.1 | 2.1:1 |
| 70-79 | 130 | 317.9 | 2.4:1 |
| 80+ | 180 | 418.8 | 2.5:1 |
Sources: NHANES autoantibody data, mortality statistics, electronic health record analysis4 5 2
2. Biological Mechanisms Driving Late-Onset Autoimmunity
Immunosenescence: The Foundation of Immune Dysregulation
Immunosenescence describes the progressive deterioration of immune function with aging, characterized by two paradoxical phenomena: immune deficiency and chronic inflammation. The thymus involutes significantly after age 50, reducing naïve T cell output by 90%, which compresses T cell receptor (TCR) diversity and impairs responses to novel antigens. Simultaneously, memory T cells accumulate and undergo senescence, marked by loss of costimulatory molecules CD27 and CD28, reduced IL-2 production, and increased secretion of pro-inflammatory cytokines including TNF-α and IL-6.6 7 8
This senescent T cell population develops a senescence-associated secretory phenotype (SASP), perpetuating inflammation through autocrine and paracrine signaling. The resulting "immune paralysis" paradoxically coexists with hyperinflammatory states, creating fertile ground for loss of self-tolerance.6
Inflammaging: The Chronic Inflammatory State
Inflammaging represents a persistent, low-grade inflammatory condition that accelerates after 50, driven by accumulated senescent cells secreting IL-1, IL-6, and TNF-α. These cytokines directly damage B cells, reducing protective antibody production while promoting autoantibody generation. Epigenetic modifications in immune cells further exacerbate this process, with age-related demethylation of apoptosis-related genes in naïve CD4+ T cells increasing autoreactivity.6 7 8
The inflammatory burden manifests systemically: C-reactive protein levels rise, endothelial dysfunction increases, and insulin resistance develops—all of which create a pro-autoimmune microenvironment.8
3. The Menopause-Autoimmune Nexus
Estrogen Withdrawal and Immune Dysregulation
Menopause represents a critical inflection point, with estrogen levels declining precipitously after age 50. Estrogen receptors exist on nearly all immune cells, regulating inflammatory gene expression. The loss of estrogen's immunomodulatory effects triggers several pathogenic cascades:1
- Cytokine Polarization: Estrogen deficiency elevates IL-1, IL-6, IL-17A, and TNF-α while reducing anti-inflammatory mediators, shifting the immune balance toward Th17 and away from regulatory T cells.1
- B Cell Dysregulation: Age-associated B cells (ABCs) expand significantly in postmenopausal women, particularly in those with rheumatoid arthritis (RA) and lupus. These cells display extensive somatic hypermutation and secrete pathogenic autoantibodies.6
- Gut Permeability: Declining estrogen and progesterone compromise intestinal barrier integrity, promoting "leaky gut" and exposing the immune system to autoantigens.9
Clinical Manifestation Patterns
The relationship between menopause and specific autoimmune diseases follows distinct patterns:
- Rheumatoid Arthritis: Disease onset peaks during perimenopause, with estrogen deficiency enhancing the pro-inflammatory activity of anti-citrullinated protein antibodies (ACPAs) through altered Fc glycan sialylation. First-degree relatives of RA patients show increased ACPA positivity following menopause.1
- Systemic Lupus Erythematosus: While SLE often presents earlier, menopause exacerbates disease activity and increases damage accrual rates.1
- Autoimmune Thyroiditis: Hashimoto's thyroiditis demonstrates a clear postmenopausal surge, with thyroid autoantibodies present in 25% of older women versus 10% of younger adults.5
4. Sex-Specific Risk Architecture
The Female Predominance
Women constitute 63% of the autoimmune disease population, with some estimates suggesting they are twice as likely as men to develop these conditions. This disparity intensifies after 50, when the female:male ratio increases from 1.5:1 in the 40-49 cohort to 2.5:1 in those over 80.2 3
The X chromosome carries numerous immune-related genes, and X-linked mosaicism may contribute to heightened autoimmunity risk. However, the post-50 acceleration primarily reflects hormonal rather than genetic factors, as evidenced by the temporal correlation with menopause.1
Premature Ovarian Insufficiency as a Model
Women with premature ovarian insufficiency (POI) before age 40 demonstrate accelerated autoimmune development, with 24-73% developing autoantibodies and increased rates of thyroiditis (27%), diabetes (2.5%), and other autoimmune conditions. This suggests estrogen withdrawal, rather than chronological aging alone, drives immune dysregulation.1
5. Environmental and Temporal Factors
Cumulative Antigen Exposure
Lifelong exposure to pathogens, chemicals, and self-antigens creates a state of chronic antigenic stress that exhausts immune tolerance mechanisms. Latent viral infections, such as cytomegalovirus, expand senescent T cell clones, reducing repertoire diversity and increasing autoreactivity.6 7
Secular Trends
Autoimmune disease prevalence has increased 50% over the past 25 years, particularly in those over 50. This temporal rise suggests environmental contributors—dietary changes, increased chemical exposures, and altered microbiome composition—interact with age-related immune vulnerability.10 11 12
6. Clinical Implications and Risk Stratification
High-Risk Phenotypes
Clinicians should identify patients exhibiting:
- Positive autoantibody screens (ANA, RF, ACPAs) without overt disease
- Chronic inflammatory markers (elevated CRP, ESR)
- Hormonal transitions (perimenopause, oophorectomy)
- Family history of autoimmunity
- Environmental exposures (smoking, silica, solvents)
Surveillance Protocols
For individuals over 50, particularly women, annual screening should include:
- Comprehensive autoantibody panel
- Thyroid function testing
- Inflammatory marker assessment
- Assessment of menopausal status and hormone therapy
7. Prevention and Management Strategies
Hormone Replacement Therapy (HRT)
The relationship between HRT and autoimmunity is complex. Estrogen therapy may modulate immune function, but effects vary by disease type, hormone formulation, and timing of initiation. In RA, early menopausal HRT may reduce risk, while in SLE, estrogen-containing contraceptives can exacerbate disease.1 11
Lifestyle Interventions
Evidence-based approaches to mitigate inflammaging include:
- Diet: Anti-inflammatory diets rich in omega-3 fatty acids and polyphenols
- Exercise: Regular physical activity reduces inflammatory cytokines and maintains immune homeostasis
- Stress Management: Chronic stress elevates cortisol and inflammatory mediators
- Sleep Optimization: Poor sleep quality accelerates immunosenescence
Emerging Therapies
Senolytic drugs targeting senescent immune cells and epigenetic modifiers represent promising frontiers. Metformin and rapamycin analogs show potential in modulating immunometabolic pathways.7
Conclusion
The sharp rise in autoimmune diseases after 50 reflects a fundamental remodeling of immune regulation driven by immunosenescence, estrogen withdrawal, and cumulative inflammatory burden. This age-associated transition creates a critical window for preventive intervention. Healthcare systems must implement age-appropriate screening protocols and personalized risk stratification, particularly for postmenopausal women who bear the brunt of this epidemiological shift. Future research must elucidate the precise molecular mechanisms linking hormonal status to immune tolerance, paving the way for targeted immunomodulatory strategies that preserve healthspan in aging populations.6 1 2 7
Related in VitaminDWiki
- Number 1 Deficiency in ALL Autoimmune Conditions (Vitamin D) - video
- Perhaps getting Vitamin D as infant decreases risk of Autoimmune Diseases as adult
- Autoimmune and high-dose vitamin D (Dr. Coimbra) - Dr. Mahtani video and transcript
- Autoimmune disease treated by Vitamin D, Zinc (and other activators of Vitamin D Receptor)
-
https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2809056
-
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.942796/full
-
https://nyulangone.org/news/aarp-risks-common-autoimmune-diseases-increase-older-people
-
https://www.palomahealth.com/learn/menopause-trigger-autoimmune-conditions
-
https://www.wsj.com/health/wellness/autoimmune-diseases-increase-age-41733014
-
https://www.aarp.org/health/conditions-treatments/info-2021/autoimmune-diseases-rising.html
-
https://www.sciencedirect.com/science/article/abs/pii/S1568997211002680
-
https://community.the-hospitalist.org/content/around-5-us-population-diagnosed-autoimmune-disease
-
https://www.autoimmuneinstitute.org/research_updates/how-aging-exacerbates-autoimmune-disease/
