Calcidiol (Calcifediol, ampli-D) approved as food supplement by EFSA – (10 micrograms per day)
News Release
Apparently to be sold by DSM as a nanoemulsion liquid
(Note: a person can take any number of drops)
Studies that supported this proposal had used 5 to 50 micrograms per day
Note: All studies were funded by DSM
EFSA assumes that it has 5X the response of Vitamin D, so:
10 micrograms of ampli-d = 50 micrograms of Vitamin D = 2,000 IU of Vitamin D
Any larger amounts in combination with other food sources would exceed ‘suitable target value’ of 4,000 IU
A few study subjects given 20 micrograms had vitamin D levels that exceeded 80 mg/mL
Spanish trial used dosings 25x and 50X larger than approved by EFSA
Note: Breast milk cannot use this form of vitamin D, but the EFSA approved it anyway for lactating women
-
Items in both categories Calcidiol and Virus:
{category}
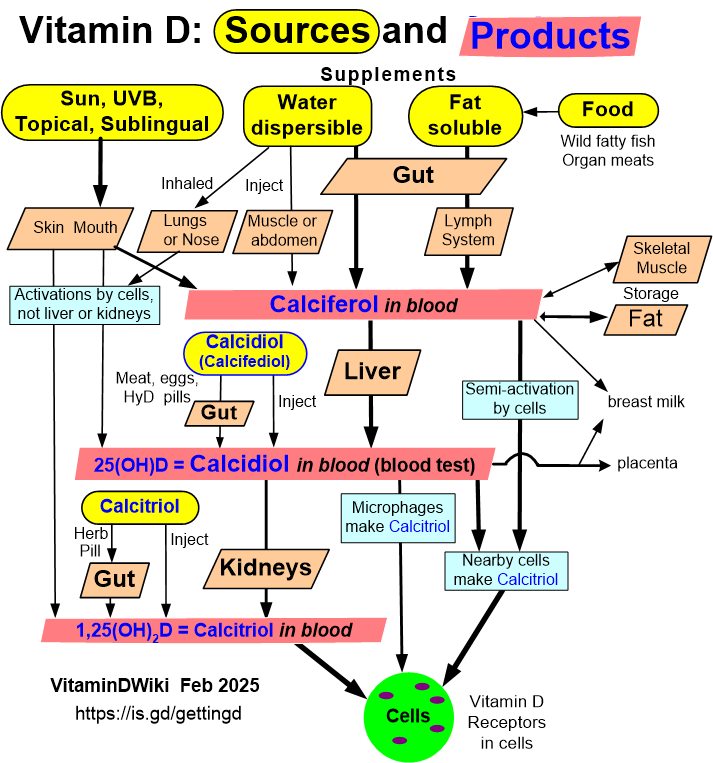
Getting Vitamin D into your blood and cells has the following chart
Safety of calcidiol monohydrate produced by chemical synthesis as a novel food pursuant to Regulation (EU) 2015/2283
EFSA Journal 2021;19(7):6660, 30 pp. https://doi.org/10.2903/j.efsa.2021.6660
EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch-Ernst, John Kearney … See all authors
Requestor: European Commission. Question number: EFSA-Q-2018-00514
Panel members: Dominique Turck, Jacqueline Castenmiller, Stefaan De Henauw, Karen Ildico Hirsch-Ernst, John Kearney, Helle Katrine Knutsen, Alexandre Maciuk, Inge Mangelsdorf, Harry J McArdle, Androniki Naska, Carmen Pelaez, Kristina Pentieva, Alfonso Siani, Frank Thies, Sophia Tsabouri and Marco Vinceti.
Acknowledgements: The Panel wishes to thank the following for the support provided to this scientific output: the former member of the EFSA Novel Foods Working Group Yolanda Sanz for the contribution provided to the development of the opinion until 3 January 2020, the members of the EFSA SCER Cross-cutting WG nanotechnologies and EFSA staff members Gabriela Precup and Patricia Romero Fernández.
Adopted: 25 May 2021
Following a request from the European Commission, the EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) was asked to deliver an opinion on the safety of calcidiol monohydrate as a novel food (NF) pursuant to Regulation (EU) 2015/2283, including its bioavailability as a metabolite of vitamin D3 when added for nutritional purposes to food supplements. The NF is produced chemically. It is proposed in food supplements up to 10 μg/day for individuals ≥ 11 years of age, including pregnant and lactating women and up to 5 μg/day in 3- to 10-year-old children. The production process, composition, specifications and stability of the NF do not raise safety concerns. Animal and human data indicate efficient absorption. The NF contains a fraction of nanoparticles, which are fat-soluble and unlikely to reach systemic distribution. There are no concerns regarding genotoxicity. Human adult studies do not raise safety concerns. Combined intake estimates of calcidiol from the NF and calcidiol and vitamin D from the diet were below the tolerable upper intake level (UL) for vitamin D for subjects above 11 years of age. The achieved mean serum 25(OH)D concentration in adults supplemented with 10 μg NF per day remained below 200 nmol/L. The Panel concludes that the NF is safe under the proposed conditions of use and use levels for individuals ≥ 11 years old, including pregnant and lactating women. The applicant did not provide data on the bioavailability and safety of the NF in children. The combined intake estimation in children (3–10 years) is close to the UL for vitamin D. Therefore, the Panel could not conclude on the safety of consumption of the NF in children (3–10 years) at the proposed daily intake. The NF is a bioavailable source of the biologically active metabolite of vitamin D, i.e. 1,25-dihydroxyvitamin D.
📄 Download the PDF from VitaminDWiki
Portion of Table of Contents
Introduction
Identity of the NF
Production process
Compositional data
Stability
Specifications
History of use of the NF and/or of Its source
Proposed uses and use levels and anticipated intake
Target population
Proposed uses and use levels
Combined intake from the NF and other sources
Absorption, distribution, metabolism and excretion (ADME)
Data on bioavailability in humans
Data on bioavailability in animals
Conclusion on ADME
Nutritional information
Conclusion on nutritional information
Toxicological information
Genotoxicity
Acute toxicity
Subacute and subchronic toxicity
Chronic toxicity, carcinogenicity, reproductive and developmental toxicity....
Human data
Allergenicity
Discussion
